When it comes to cbd products, the FDA and FTC have issued several warning letters about the industry's marketing practices. These letters focused specifically on products whose claims were not approved by companies. They also highlighted unapproved new drugs introduced without FDA approval as they were not FDA-approved. Curaleaf and Advanced Spine and Pain, LLC, received the letters.
FD&C Act
The FD&C Act regulates how food and other products are sold. It was passed to protect interstate commerce from the sale or distribution of adulterated or mislabeled food. The Federal Food, Drug, and Cosmetic Act was passed by Congress, which gave the FDA the authority to regulate medical products. It also banned misbranding of cosmetics or therapeutic medical devices. Other important laws of the twentieth century were the Public Health Service Act as well the Radiation Control and Health and Safety Act.
The FD&C Act also protects consumers by requiring the manufacturer to disclose adverse events. FDA may recall devices that are not compliant with these regulations. The FDA can impose civil sanctions on the company, or even require it to remove the product completely from the market.

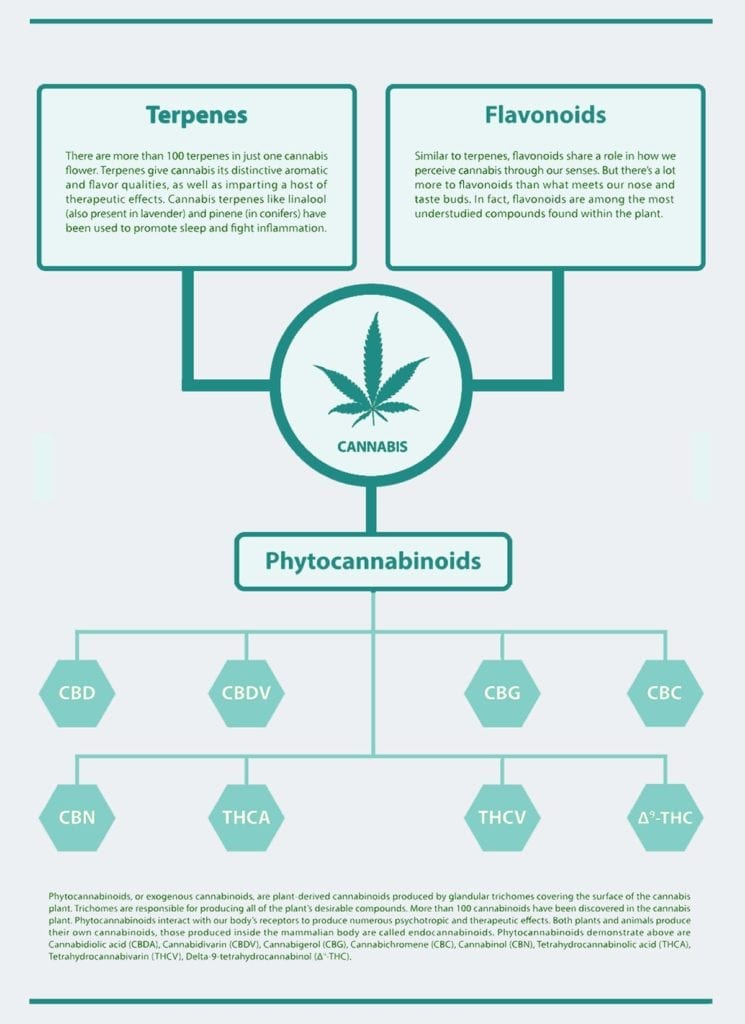
Cannabidiol is a pharmacological agent
Cannabidiol is an important component of cannabis and has several potential clinical uses. Its pharmacological activity has been demonstrated in both animals as well as humans. It is well-known for its anti-inflammatory as well as analgesic properties. This makes it a potential treatment for inflammatory conditions, such pain and inflammation.
Cannabinoids reduce the behavioral responses to noxious stimuli, in animal and human models of pain. Cannabinoids work on CB2-like nerve receptors. These receptors are found in the spinal cord and ventro-posterolateral nucleus of the thalamus.
Safety risk for animals
Despite the positive effects for human health, FDA is still concerned over the safety of CBD products in the hands of food-producing mammals. Despite not testing CBD products for animal use in any way, the FDA has repeatedly sent warning letters out to CBD-selling companies. In addition, the FDA has not yet evaluated the safety of CBD products, including their proper dosage and interactions with other FDA-approved drugs.
FDA safety concerns regarding CBD in food-producing animal production stem from a lack safety data and efficacy. FDA is concerned that animal owners may delay treatment due to reliance on unproven CBD claims. CBD food may also contain heavy metals and pesticides that have not been reviewed by FDA.
The regulatory framework for products containing CBD
The FDA must develop a clear regulatory framework in order to make decisions about the safety and quality CBD products. The regulatory framework should give an overview of the latest scientific data and provide a roadmap for filling in any data gaps. It should include requirements that CBD products be notified to FDA in order for FDA review. The framework should also offer opportunities for stakeholders and information sharing.
CBD products can be regulated by both the federal and state governments in different ways. CBD products in many states have not been approved by the FDA and are subject to rigorous regulatory enforcement. In addition, ongoing litigations are ongoing regarding CBD products' legality. These laws are important to ensure that companies do not become liable for a lawsuit.
FAQ
What is the future in CBD?
The future is bright for the CBD industry. It's easy for people to get on board with this sector. This market is expanding exponentially with CBD products being purchased globally at a total of $1 billion.
Statista reports that in 2019, global sales of CBD (cannabidiol) are expected to exceed $22.4 Billion. This is an almost 200% increase from 2018!
Also, the CBD market is expected to grow at a compound annual rate of 22.5%. This would translate to almost $6.8 billion in revenues by 2022.
This is great news not only for existing businesses but also for companies looking to get into the sector. But, it is important to remember that the CBD industry is still in its infancy. There will be many challenges.
What are some common blunders that companies make when they venture into the US cannabinoid markets?
Not understanding the regulations for cannabis products is a first mistake. This could cause you to have to modify the formulation of your product.
Unskilled labeling is the second. You must know whether your product contains CBD, THC, or both.
Finally, you must know how to package your product correctly. If your product contains THC, you need to ensure it is packed in child-resistant containers.
You can still use all the packaging laws even if your product contains no THC. Many states have legalized cannabidiol (CBD).
Remember to keep track of any recalls for your products. Customers should be notified as soon as possible if there are any issues with their product.
Is there a CBD industry that is growing?
The answer is yes! As legalization spreads throughout North America, this growth is expected continue. Canada is the only country that has legalized recreational use of cannabis. Many states have also passed medical marijuana laws.
As more states adopt legislation that allows medicinal marijuana access, this trend is likely to continue at least for the next decade.
Economically, legalizing marijuana makes economic sense. Legalizing marijuana has many other benefits.
It could help decrease crime rates by reducing illegal drug availability. It could also be a source for tax revenue.
People may choose to drink less alcohol as legal marijuana becomes more popular. This would mean fewer hangovers and lower health care costs.
Chronic pain sufferers may find that marijuana can actually improve their quality of life. Many believe THC, the active component in marijuana, is responsible for relieving symptoms like muscle spasms or nausea that can be caused by chemotherapy.
It is possible that marijuana could be used to treat mental disorders such as anxiety or depression. Some studies have shown that marijuana can treat schizophrenia.
Even though the CBD industry looks promising, there are still many challenges to be overcome.
Can I use CBD during pregnancy?
It is not clear if CBD is safe for use during pregnancy.
Based on the limited information, however, it seems unlikely that CBD would cause harm for the baby.
It is important to remember that CBD should not only be used by women who are pregnant, but also by those who have been recommended by their doctor.
The Food and Drug Administration published a warning about potential health risks when CBD is taken while pregnant.
According to the FDA, "there is some evidence that cannabis use during pregnancy may increase the risk of miscarriage."
The agency added that more research is needed before a firm conclusion can be drawn.
Statistics
- The inhibition of FAAH is predicted to lead to an increase in brain and plasma concentrations of AEA, which acts as a partial agonist at CB1R and CB2R, thereby increasing endocannabinoid tone [92, 110]. (ncbi.nlm.nih.gov)
- CBD seems unlikely to directly influence sleep in healthy humans [115] (and maybe “sleep-promoting” in those with certain comorbid conditions) (ncbi.nlm.nih.gov)
- however, one study also found that these effects were virtually abolished when the original media (a nutrient broth agar) was replaced with one containing 5% blood (increasing the minimum concentration to ~160 μM CBD) [179]. (ncbi.nlm.nih.gov)
- As a substance that was federally illegal before the passage of the 2018 Farm Bill, hemp-derived cannabinoids with no more than 0.3% THC still face a regulatory grey area. (forbes.com)
- The use of these products is likely to become even more widespread if the World Health Organization's recommendation that CBD no longer is scheduled in the international drug control conventions is adopted by the United Nations member states [201]. (ncbi.nlm.nih.gov)
External Links
How To
What are the issues that the CBD industry faces?
The current market for CBD-based products is expanding at a phenomenal rate. There are many hurdles businesses face when trying to enter the CBD market. These include a lack consumer awareness, high-cost entry, limited access capital and regulatory uncertainty.
Many consumers do not know what CBD is or how it works. This makes it difficult for consumers to make informed decisions on whether or not they want CBD products.
As a result, most CBD companies rely heavily on word-of-mouth marketing. This is costly because they have to pay for advertising and hire staff to promote their brand.
High production costs are another problem facing new entrants in the CBD industry. The raw materials needed to create CBD products are quite expensive. CBD oil is made from hemp that has been grown in particular climates.
Grow enough hemp to produce CBD oil requires approximately $1,000 per annum. As a result, many small farmers cannot afford to start.
A lack of capital access is another issue that new entrants will face in the CBD marketplace. Banks discourage many people from starting a business because of the stigma attached to this industry.
The sale of CBD products is still subject to regulatory uncertainty. There are currently no clear guidelines regarding how CBD products should be marketed.
Despite some states having passed laws restricting the sale CBD products, this is not yet a national policy.
Only Nevada, Maine, and Nevada have legalized recreational pot.
Massachusetts and Michigan are however considering similar measures.
These changes could result in increased competition between CBD manufacturer.
These factors are why many entrepreneurs prefer to work from home than open a physical store.